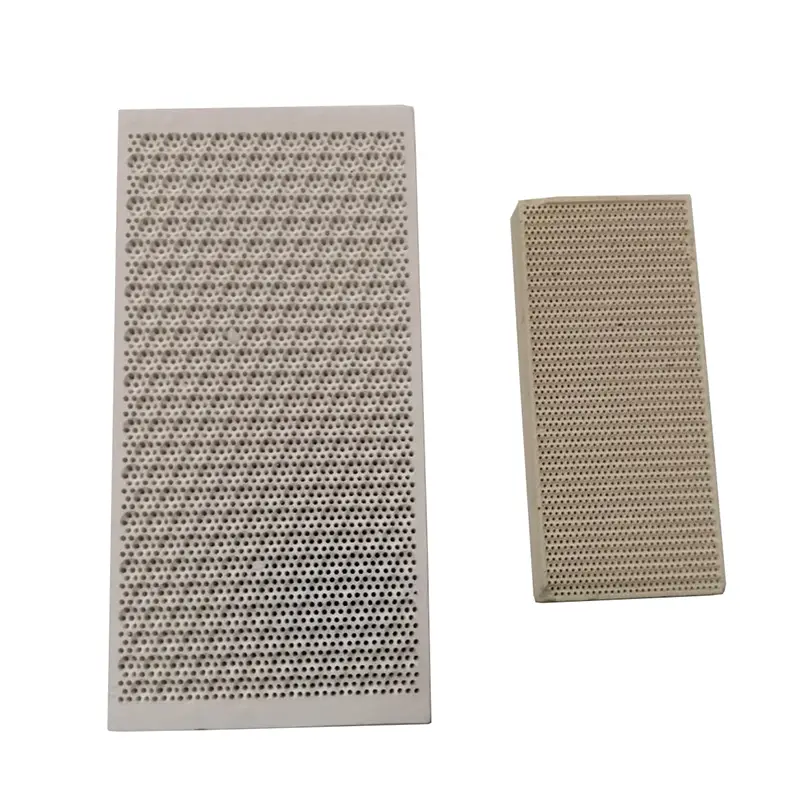
Introduction and application of Steatite Ceramics
I. Main Composition of Steatite Ceramics
The primary raw material is talc, typically accounting for 60–95% of the formulation. At high temperatures (>1000°C), talc decomposes to form enstatite (MgSiO₃), which provides low dielectric loss and high mechanical strength.
Sintering aids include:
-
Clay (e.g., kaolin, 5–15%) – Improves plasticity and sintering activity.
-
Feldspar (e.g., potassium feldspar, 3–10%) – Lowers sintering temperature.
-
Alumina (Al₂O₃) – Enhances hardness and thermal stability.
-
Other oxides (e.g., ZrO₂, TiO₂) – Modifies grain boundary structure or suppresses abnormal grain growth.
II. Crystal Structure of Talc
Structurally, talc consists of layered magnesium silicate sheets. Each sheet is composed of:
-
Silica tetrahedra (SiO₄): Each silicon atom bonds with four oxygen atoms, forming a tetrahedral structure.
-
Magnesium ions (Mg²⁺): Positioned between silica chains, linking the layers.
These silica-magnesium layers are stacked together via weak van der Waals forces, allowing talc to exhibit perfect cleavage—meaning it can be easily split or peeled into thin flakes along the interlayer direction.
Key Features of Steatite Ceramics
-
Low dielectric loss (ideal for insulators)
-
High mechanical strength
-
Good thermal shock resistance
-
Chemically inert (resists acids/alkalis)
-
Cost-effective compared to advanced ceramics
II. Manufacturing Process of Steatite Ceramics
The production of steatite ceramics involves several key steps, including raw material preparation, forming, sintering, and post-processing. Below is a detailed breakdown of the process:
1. Raw Material Preparation
-
Talc Selection & Purification
-
High-purity talc (Mg₃Si₄O₁₀(OH)₂) is selected to minimize impurities (e.g., Fe₂O₃, CaO).
-
Purification may involve flotation or acid washing to remove contaminants.
-
-
Additive Mixing
-
Clay (kaolin), feldspar, and oxides (Al₂O₃, ZrO₂) are added to improve sintering and mechanical properties.
-
The mixture is ball-milled to ensure homogeneity (particle size <10 μm).
-
2. Forming
Common shaping methods include:
-
Dry Pressing (Most common for industrial parts)
-
Powder is compacted under high pressure (50–150 MPa) using hydraulic presses.
-
Binders (e.g., PVA) may be added to enhance green strength.
-
-
Extrusion (For tubes or rods)
-
Plasticized mixture (with water/organic binders) is forced through a die.
-
-
Slip Casting (For complex shapes)
-
Aqueous slurry is poured into plaster molds, where water is absorbed, leaving a solid layer.
-
3. Drying
-
Formed "green" bodies are dried at 80–120°C to remove moisture.
-
Controlled drying prevents cracking due to shrinkage.
4. Sintering
-
Fired in electric or gas kilns at 1250–1400°C (depending on composition).
-
Phase Transformation: Talc (Mg₃Si₄O₁₀(OH)₂) decomposes to enstatite (MgSiO₃) at ~1000°C, enhancing strength.
-
Densification: Liquid phase (from feldspar) aids particle bonding at high temperatures.
5. Post-Processing (Optional)
-
Machining: Grinding/lapping to achieve precise dimensions.
-
Glazing: For aesthetic or waterproofing purposes (e.g., insulators).
-
Metallization: Electrode deposition (for electronic components).
Key Process Considerations
-
Talc Particle Size: Finer particles improve sinterability but may increase shrinkage.
-
Sintering Atmosphere: Air (oxidizing) or nitrogen (for reducing oxidation risks).
-
Cooling Rate: Slow cooling minimizes thermal stresses.
Comparison with Other Ceramics
Property Steatite Alumina (Al₂O₃) Zirconia (ZrO₂) Dielectric Loss Very Low Moderate High Strength (MPa) 100–200 300–500 800–1200 Cost Low Medium High Steatite’s balance of performance and cost makes it ideal for insulators, electronic substrates, and precision components.
-