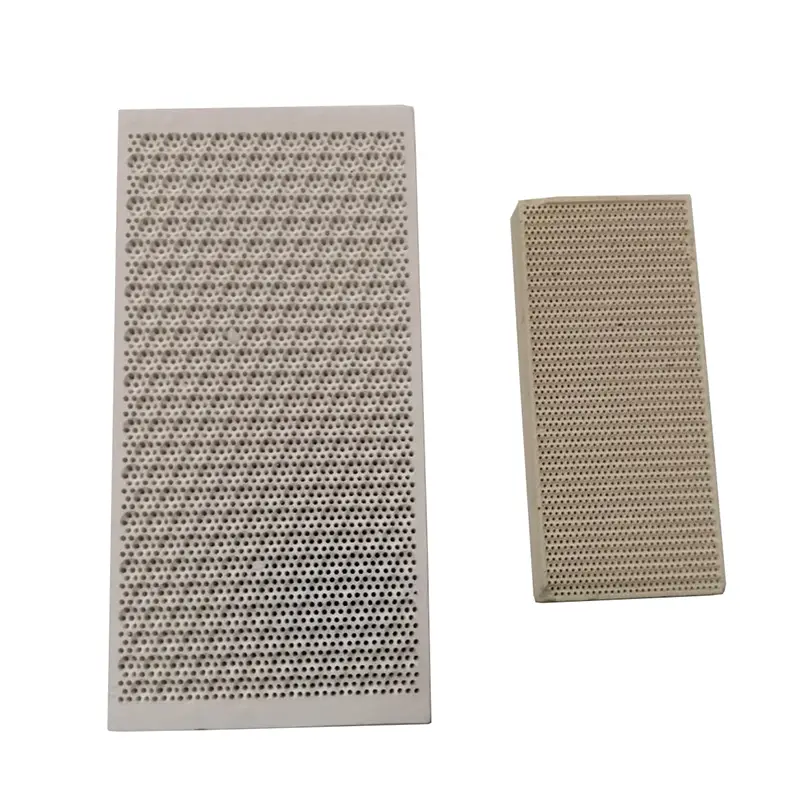
What is cordierite ceramic ?
Cordierite Ceramics are well known for their low thermal expansion and refractory character. Although cordierite is available as a powder, here we are generally talking about ceramic that went into the kiln as an ordinary composite of mineral powders but emerges as a solid cordierite crystalline matrix (grown by the firing process tuned to its needs).
Cordierite makes the ceramic manufacture of products like catalytic converters possible. They constantly heat up and cool down quickly and must not crack because of the shock. These converters get their thermal shock resistance properties from a bonded matrix of low-expansion cordierite crystals.
Cordierite crystals form during firing between 1200-1400C (~cone 6-15). Interestingly, cordierite crystals have a lesser thermal expansion along one axis than another. The ideal chemistry to produce the crystals is 13.8% MgO, 34.8% Al2O3 and 51.4% SiO2 (2MgO, 2Al2O3, 5SiO2). Talc, kaolin and raw alumina powder can be blended to source this chemistry (other materials are also used e.g. aluminum hydroxide, steatite and other MgO minerals). Multiple factors determine the temperature and extent to which cordierite develops. Materials sourcing more than one oxide (like kaolin and talc) may react better than pure oxide materials (like MgO, Al2O3, SiO2). Materials of finer particle size react better. Better cordierite development occurs when the precursor mix is pressed to higher densities and firing schedules are tuned (in rate-of-climb, ultimate temperature and holding patterns). Thermal expansion measuring equipment is needed to study the relationships between materials, process and firing on the quality of cordierite produced. As noted, the ultimate goal is the development of a continuous crystal matrix of cordierite (not just the presence of the crystals in an otherwise typical ceramic).